Digestion of protein: microbes
Class practical
This activity involves skills for safe handling of microbial material. Students compare the activity of an enzyme extract with that of two enzyme-producing microbes and can consider the industrial importance of enzymes and microbes.
![]() This practical is based on an investigation called
This practical is based on an investigation called ![]() Breakdown of protein by microbes (319 KB) published in Practical Microbiology for Secondary Schools © Society for General Microbiology.
Breakdown of protein by microbes (319 KB) published in Practical Microbiology for Secondary Schools © Society for General Microbiology.
Lesson organisation
Give each working group (1-2 students) a milk-agar plate. They will have to use sterile paper discs to collect samples of two microbes and one enzyme extract, all using sterile technique. Organise the work so that not all the groups want bottle A at the same time!
Written information about industrial processes using microbes and enzymes (particularly proteases) would be a useful resource for this lesson. See links at end.
There is scope here for developing skills of sterile technique to become the focus as well as exploring the enzyme activity.
Apparatus and Chemicals
For each group of students:
Forceps (1)
Sterile paper discs (4)
Ruler (1)
Marker pens to label the plates
Tape to close up the plates (Note 2)
For the class – set up by technician/ teacher:
Bacillus subtilis nutrient broth culture, 2 cm3 in a bottle/ test tube
Saccharomyces cerevisiae malt extract broth culture, 2 cm3 in a bottle/ test tube
Milk-agar plates, 2 per group (Note 1)
Virkon solution 1% w/v (see manufacturer’s instructions)
Bottles of sterile distilled water, 1 per 4 groups
Bottles of 0.1% trypsin solution, 1 per 4 groups
Health & Safety and Technical notes
Carry out a full risk assessment before planning any work in microbiology (Note 1 for more details).
Check the standard procedures for more details of Maintaining and preparing cultures, Aseptic techniques, Making up nutrient agars, Pouring an agar plate and Incubating and viewing plates safely.
1 Before embarking on any practical microbiological investigation carry out a full risk assessment. For detailed safety information on the use of micro-organisms in schools and colleges, refer to Basic Practical Microbiology – A Manual (BPM) which is available, free, from the Society for General Microbiology (email This email address is being protected from spambots. You need JavaScript enabled to view it.) or go to the safety area of the SGM website (http://www.microbiologyonline.org.uk/teachers/safety-information) or refer to the CLEAPSS Laboratory Handbook.
2 To make milk-agar, make up and sterilise nutrient agar. Allow to cool to 40-50 ºC and add pasteurised milk (10% by volume) aseptically and mix carefully. The milk should be pasteurised and freshly bought but can be skimmed, semi-skimmed or full-cream. See Standard procedures: Making up nutrient agars for more details.
3 Make up 0.1% trypsin solution. Refer to CLEAPSS Hazcard and Recipe card. Powdered enzyme is harmful, but solutions less than 1% are considered LOW HAZARD. Make up fresh for each day of use as it degrades over time.
4 Make your own assay discs by punching discs around 6mm diameter from filter paper, or chromatography paper using a cork borer or a hole punch. Wrap in aluminium foil, in sets of four, and sterilise by autoclaving.
Procedure
SAFETY: Use sterile technique when handling microbial cultures. Incubate and view plates safely. Sterilise and/ or dispose of all contaminated material using appropriate methods.
Preparation
a Inoculate nutrient broth using sterile technique from a clean culture of Bacillus subtilis at least 48 hours before use. Inoculate malt extract broth with Saccharomyces cerevisiae at the same time. (See Standard procedures: Maintaining and preparing cultures of bacteria and yeasts)
b Calculate the quantity required and prepare just enough milk-agar (Note 2) for the investigation (12-15 ml for normal depth in a 90 mm Petri dish).
c Prepare a suitable solution to disinfect the work area for the investigation and afterwards. Suitable disinfectants include sodium chlorate(I) (hypochlorite) at concentrations providing 1000 ppm available chlorine for general surface cleaning, or 2 500 ppm chlorine for discard pots, or VirKon (follow manufacturer’s instructions).
d Prepare 0.1% trypsin solution (Note 3).
e Sterilise distilled water in enough McCartney/ Universal bottles.
f Paper discs can be purchased (Whatman Antibiotic Assay discs) or you can make your own (Note 4).
Investigation
a Keep one milk-agar plate unopened as a control.
b Provide each working group with one milk-agar plate.
c Students use a marker pen to mark four sections on the base. Label the sections A, B, C, and D. Write a key to record the treatment of each disc, for example
| Area |
Treatment |
| A |
Bacillus subtilis |
| B |
Saccharomyces cerevisiae |
| C |
0.1% trypsin solution |
| D |
Distilled water |
d Pass the forceps through a bunsen flame. Allow them to cool and use them to pick up one of the paper discs.
e Open a bottle containing bacterial culture (or remove the cotton wool stopper from the test tube). Flame the neck and dip the disc into the broth. Allow excess to drip off. Flame the neck again and replace the top or stopper. Transfer the disc to the middle of the correct section on the agar plate.
f Repeat d and e using another disc and the fungal culture.
g Repeat d and e using another disc and the trypsin solution.
h Repeat d and e using another disc and the distilled water.
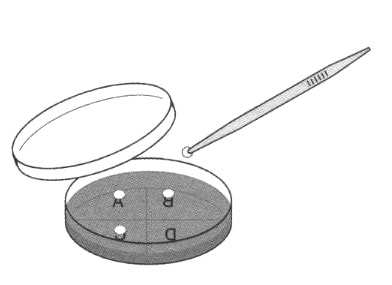
i Place the forceps in a discard beaker.
j Tape up the dish and incubate the plates (see Standard procedures).
k Put the dish on squared paper. The milk agar is usually opaque, but clears as the protein is digested. Measure and record the diameter of any clear zones around the discs. A wider diameter suggests a greater concentration of protease. Do not open the plates.
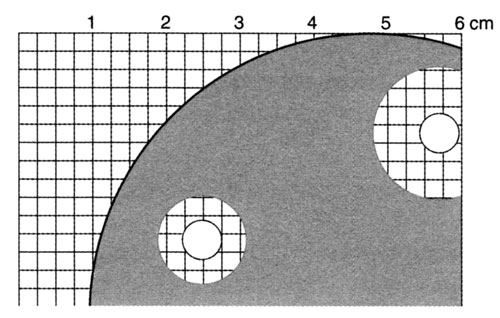
Alternative procedure – without paper discs
l Remove microbial culture or enzyme solution from stock using a Pasteur (dropping) pipette and aseptic technique.
m Place one drop of microbial culture (or enzyme solution) in the centre of the marked area of the agar plate.
n Keep the plate upright until the drops have dried, then invert.
Teaching notes
This protocol introduces a quantitative assay technique for enzyme activity. It is worth discussing the value and limits of this as a technique.
The fact that enzymes (of microbial origin) will hydrolyse proteins to produce amino acids is industrially important in detergent manufacture, brewing and baking, meat tenderisation and leather preparation. A text-based exercise, such as gathering useful information and making notes from a complex source, would be a useful companion to this practical work.
The milk agar is opaque due to the milk protein casein. After inoculation and incubation, clear areas around the microbial colonies, or introduced enzyme indicate protease activity. Assume that the microbial population in the milk has no effect on the outcome of the investigation. The control plate and the central areas of the dish will show this if is true.
Compare with the protocol for digestion of starch by microbes.
Health & Safety checked, May 2009
Downloads
Download the student sheet ![]() Digestion of protein: microbes (180 KB) with questions and answers.
Digestion of protein: microbes (180 KB) with questions and answers.
Weblinks
www.microbiologyonline.org.uk/teachers/resources
Society for General Microbiology – source of Basic Practical Microbiology, an excellent manual of laboratory techniques and Practical Microbiology for Secondary Schools, a selection of tried and tested practicals using microorganisms. The protocol is based on Breakdown of protein by microbes.
www.microbiologyonline.org.uk
MiSAC (Microbiology in Schools Advisory Committee) is supported by the Society for General Microbiology (see above) and their websites include more safety information and a link to ask for advice by email.
www.ncbe.reading.ac.uk/NCBE/PROTOCOLS/pracbiotech.html
The NCBE is a rich source of up-to-date protocols and practical equipment for biotechnology practicals in schools. These notes (from 1993) cover enzyme reactions processing sugars in Better milk for cats and Glucoamylase production by yeast. The latter uses a bioreactor in which yeast ferments a starch suspension that can be sampled periodically.
www.lsbu.ac.uk/biology/enztech/proteases.html
One example of the kind of information available about protease enzymes and industrial processes.
(Websites accessed October 2011)


