Incubating and viewing plates
Incubating the plates to promote growth of microbes is an essential part of any microbiology investigation. Incubating in aerobic conditions, and below human body temperature, reduce the risk of encouraging microorganisms (particularly bacteria) that could be pathogenic to humans. Taping the lids on reduces the chance that students will open plates when viewing, but there are details below of how to kill plates completely if this is still a significant risk.
Health & Safety and Technical notes
Carry out a full risk assessment before starting any microbiology work (see note 1 for more details).
1 Before embarking on any practical microbiological investigation carry out a full risk assessment. For detailed safety information on the use of microorganisms in schools and colleges, refer to Basic Practical Microbiology – A Manual (BPM) which is available, free, from the Society for General Microbiology (email This email address is being protected from spambots. You need JavaScript enabled to view it.) or go to the safety area of the SGM website (www.microbiologyonline.org.uk/safety.html) or refer to the CLEAPSS Laboratory Handbook, section 15.2.
2 Keep plates at room temperature or incubate at 20-25 °C for 2-3 days. Fungi grow more successfully at lower temperatures. Do not incubate at human body temperature (or above 30 °C) – this reduces the risk of culturing microbes that are pathogens to humans.
3 Reducing the temperature to 4 °C will slow the growth of any cultures – so you can show your students a 2-3 day growth if your lessons are a week apart.
4 All inoculated plates must be taped before incubation to ensure they cannot be opened accidentally. Do this by fixing with 2 or 4 short strips of adhesive tape at opposite edges of the dish. Do not seal completely as this may promote the growth of anaerobic pathogens or prevent normal growth by restricting diffusion of oxygen. See CLEAPSS Laboratory Handbook 15.2.10.
5 Plates are incubated upside down (agar up), so that condensation does not drip onto the plate and interfere with the developing microbes.
6 You might replace lids if condensation makes viewing difficult, so label plates on the bottom – with Chinagraph or wax pencils, permanent marker pens or a small adhesive label at one edge.
7 Count the plates out and in again to ensure that you have collected all the plates at the end of a lesson.
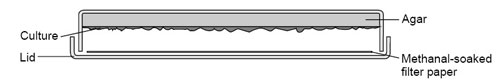
8 You can seal plates around the whole circumference just before viewing if you think there is any risk that your students will open the plates. Or you can stop the growth of a culture completely by placing a piece of filter paper into the lid of the inverted plate. Add a little 40% methanal solution carefully to soak the filter paper and replace the base. Leave for 24 hours. Remove the filter paper, remove any surplus liquid, and reseal the plate. See CLEAPSS Laboratory Handbook section 15.2.11. On Hazcard 063, methanal is described as toxic at this concentration and a category 3 carcinogen.
Web links
www.microbiologyonline.org.uk/sgmprac.htm
Society for General Microbiology – source of Basic Practical Microbiology, an excellent manual of laboratory techniques and Practical Microbiology for Secondary Schools, a selection of tried and tested practicals using microorganisms.
www.microbiologyonline.org.uk
MiSAC (Microbiology in Schools Advisory Committee) is supported by the Society for General Microbiology (see above) and their websites include more safety information and a link to ask for advice by email.
(Websites accessed October 2011)


