Hazards of defrosted food
Class practical
This investigation shows the rate of bacterial growth in uncooked vegetables, using a routine method to estimate microbial numbers (the Miles & Misra technique). Find out what happens to the microbes on frozen peas when the peas are stored after defrosting.
 This practical is based on an investigation called
This practical is based on an investigation called ![]() Microbes and food spoilage published in Practical Microbiology for Secondary Schools © Society for General Microbiology.
Microbes and food spoilage published in Practical Microbiology for Secondary Schools © Society for General Microbiology.
Lesson organisation
Half the class could work on freshly defrosted peas, the other half on peas defrosted yesterday (‘day-old peas’). At the minimum, each working group needs a sample of 3 peas (either freshly defrosted or ‘day-old’) in a clean test-tube. If you feel it is worth the time and apparatus, each working group could investigate both kinds of peas.
Apparatus and Chemicals
For each group of students:
Calibrated dropping pipette, 1 (Note 1)
Sterile 1 cm3 syringe/ pipette and filler, 1
Sterile 10 cm3 syringe/ pipette and filler, 1
Test tube rack, 1
Test tubes, 6
Glass rod, 1
Sterile distilled water, 50 cm3
Sample of peas in a test-tube, 3, either freshly defrosted, or defrosted for 24 hours
Beaker of disinfectant (Note 2)
Nutrient agar plate, 1
Marker pen
Adhesive tape
For the class – set up by technician/ teacher:
Samples of peas in test-tubes, 3 per tube, either freshly defrosted or defrosted and left for 24 hours
Nutrient agar plates, 1 per working group (see Standard techniques)
Beakers of disinfectant
Health & Safety and Technical notes
Carry out a full risk assessment before planning any work in microbiology (see Note 1 for more details).
Check the standard techniques for more details of Aseptic techniques, Making up nutrient agars, Pouring an agar plate, and Incubating and viewing plates.
If students have sensitive skin, allow access to gloves when likely to come into contact with the disinfectant solution.
Do not eat the peas! Uncooked vegetables like this are safe to use in this way. Raw meat must not be used as it may contain pathogens.
1 Before embarking on any practical microbiological investigation carry out a full risk assessment. For detailed safety information on the use of micro-organisms in schools and colleges, refer to Basic Practical Microbiology – A Manual (BPM) available free from the Society for General Microbiology (email This email address is being protected from spambots. You need JavaScript enabled to view it.) or go to the safety area of the SGM website (www.microbiologyonline.org.uk/safety.html), or refer to the CLEAPSS Laboratory Handbook, section 15.
2 Calibrating a Pasteur pipette: You can calibrate a Pasteur pipette by drawing in a known volume (eg 1 cm3) and counting the number of drops formed as it is discharged. Plastic disposable Pasteur pipettes are available from school science suppliers. The drop size from plastic disposable Pasteur pipettes is about 0.02 cm3.
3 Suitable disinfectants include sodium chlorate(I) (hypochlorite) at concentrations providing 1000 ppm available chlorine for general surface cleaning, or 2500 ppm chlorine for discard pots, or Virkon (follow manufacturer’s instructions).
Procedure
SAFETY:
Make sure you know how to sterilise and dispose of the pea suspensions and agar plates after the investigation.
Preparation
a Prepare nutrient agar plates as described in Standard techniques: Making up nutrient agars and Pouring an agar plate.
b Ensure the glassware is as clean as possible. For best results it should be sterile.
c One day before: defrost a sample of peas.
d On the day: defrost a second sample of peas.
e Set out 3 peas in each test-tube – labelled F (‘fresh’) or ‘D’ (‘day-old).
Investigation
a Collect your sample of peas. Note whether they are freshly defrosted (F) or day-old peas (D).
b Use the 10 cm3 syringe or a pipette to add 5 cm3 of sterile distilled water to your sample of peas.
c Gently crush the peas using the glass rod. Mix them thoroughly with the water. Label A.
d Allow the mixture to settle.
e Label five test tubes: A1, A2, A3, A4, A5.
f Use the 10 cm3 syringe or pipette to put 9 cm3 of sterile water in each one.
g Using a 1 cm3 syringe or pipette, mix the contents of tube A thoroughly by filling and emptying the syringe / pipette several times.
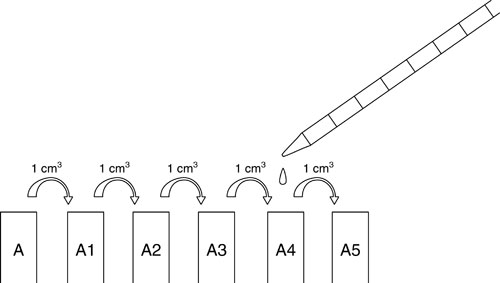
h Put 1 cm3 of well-mixed pea suspension from A into tube A1.
i Mix thoroughly by filling and emptying the syringe a few times.
j Put 1 cm3 of well-mixed solution from A1 into A2.
k Repeat for A3, A4 and A5.
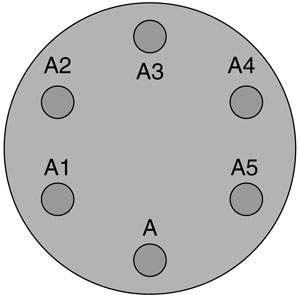
l Mark an agar plate on the bottom, as shown on the right. Label it with name, date and F or D.
m Use the calibrated dropping pipette to draw up a small amount of the sample from A5. (Note 2.)
n Lift the lid of the Petri dish. Release one drop onto the agar at position A5. Release the drop as close to the surface of the agar as possible to avoid splashing. Replace the lid.
o Return any excess solution to the A5 tube. Using the same pipette, repeat with tubes A4, A3, A2 and A1.
p Discard the pipette into a beaker of disinfectant (Note 3).
q Allow the drops to soak into the agar. Do not move the plates before the drops have soaked in, or the pea extracts will move. This may take a while.
r Tape the agar plate and incubate inverted for 2-3 days at room temperature (20-25 °C). You can then refrigerate the plates until they are examined.
s Estimate the number of bacteria in the peas using the Miles & Misra technique (see Teaching notes below).
Teaching notes
Miles & Misra technique
Drops of liquid containing large numbers of viable cells give rise to circular areas of confluent growth. Any drop containing less than about 15 viable cells will produce a small countable number of colonies.
The initial 1 cm3 of pea suspension contains about 20% of the microbes from the peas. The dilutions contain smaller amounts as shown below.
| Tube |
Percentage of total in 10 cm3 of solution in tube |
Percentage of total in 0.02 cm3 drop |
| A1 |
2 % |
0.004 % |
| A2 |
0.2 % |
0.0004 % |
| A3 |
0.02 % |
0.00004 % |
| A4 |
0.002 % |
0.000004 % |
| A5 |
0.0002 % |
0.0000004 % |
So if dilution A3 contains about 8 viable cells, and makes a small number of countable colonies (say 8), then the number you count (8) is 0.00004 % of the total number of microbes, and the total number of microbes is 8 x 100 ÷ (4 x 10-5) = 2 x 107.
You can do the calculation, or simply compare the results of the dilutions for fresh and day-old peas. If you get similar numbers of colonies from dilution A3 for fresh and A4 for day-old peas, it means there are 10 times as many microbes on the day-old peas. If A1 for fresh produces similar numbers of colonies to A5 for day-old, it means there are 10,000 times as many microbes on the day-old peas.
Web links
www.microbiologyonline.org.uk/teachers/resources
Society for General Microbiology – source of Basic Practical Microbiology, an excellent manual of laboratory techniques and Practical Microbiology for Secondary Schools, a selection of tried and tested practicals using microorganisms. This protocol is based on Microbes and food spoilage (310KB).
www.microbiologyonline.org.uk
MiSAC (Microbiology in Schools Advisory Committee) is supported by the Society for General Microbiology (see above) and their websites include more safety information and a link to ask for advice by email.
(Websites accessed October 2011)


