Investigating what makes fruit go brown
Class practical
Apples and potatoes go brown after cutting or crushing. This practical involves making observations of the process and developing (or considering) hypotheses that might explain the reaction. Each hypothesis is tested by further investigations. The conclusions have applications in commercial food processing, where it is important to reduce or prevent the browning of fruits and vegetables to maintain their appeal to consumers.
Lesson organisation
After an initial observation of browning, there are four investigations of the process of browning. All students should be involved in making initial observations, then each group of students could carry out one further investigation and pool their results for the class. This would reduce the time and materials required. Investigation 3 is quite complicated in terms of handling glassware and gases, and may be better as a demonstration.
Apparatus and Chemicals
For each group of students:
Initial observations:
Scalpel or knife to cut fruit or vegetable samples
Tiles to cut on
Mortar and pestle
Tongs or forceps, 1
Apple or potato, 1 medium sized
Distilled water
Benzene-1,2-diol solution in water, 1%, in a dropper bottle (Note 1)
Investigation 1
Tongs or forceps, 1
Beaker, 100 cm3, 2
Phenol, 1%, 50 cm3 (Note 2)
Distilled water
Benzene-1,2-diol solution in water, 1%, in a dropper bottle (Note 1)
Investigation 2
Beaker, 100 cm3, 4
Tongs or forceps, 1
Kettle, to boil water
Cold water supply – to mix with boiling water to make series of water baths
Thermometer, to read up to 100 °C, 3
Benzene-1,2-diol solution in water, 1%, in a dropper bottle (Note 1)
Investigation 3
Tongs or forceps, 1
Boiling tubes, 3
Boiling tubes with associated tubing to investigate the effect of different atmospheres (Note 3)
Carbon dioxide generator (Note 3)
Nitrogen generator (or rather oxygen absorber) (Note 3)
Benzene-1,2-diol solution in water, 1%, in a dropper bottle (Note 1)
Investigation 4
Beaker, 100 cm3, 6
Tongs or forceps, 1
Ascorbic acid solution at different strengths (5%, 3.5%, 2.5%, 2%, 1%), 50 cm3 of each
Benzene-1,2-diol solution in water, 1%, in a dropper bottle (Note 1)
Investigation 5
Beaker, 100 cm3, 7
Tongs or forceps, 1
Hydrochloric acid, 2 %, 50 cm3
Citric acid, 2%, 50 cm3
Sodium hydrogensulfate(IV) (sodium hydrogensulfite), 2%, 50 cm3
Sodium chloride, 2%, 50 cm3
Sucrose, 2%, 50 cm3
Boiling water (from kettle)
Distilled water (cold)
Benzene-1,2-diol solution in water, 1%, in a dropper bottle (Note 1)
Health & Safety and Technical notes
Do not eat any of the apple or potato.
Take care with all the solutions and hot water from the kettle.
Wear eye protection when handling the benzene-1,2-diol, phenol and acids.
Be aware of students and staff with breathing difficulties such as asthma; sulfur dioxide from the sodium hydrogensulfate(IV) might exacerbate the condition (Note 5).
1 Benzene-1,2-diol (catechol) and similar compounds in fruit and vegetables are the substances oxidised to cause the colour change. If the apple or potato is not browning quickly, add 3-4 drops benzene-1,2-diol to the sample to provide more substrate for the enzymes to work on. Benzene-1,2-diol is described on Hazcard 12 as HARMFUL in contact with the skin or if swallowed, and IRRITATING to the eyes and skin. Wear eye protection and avoid skin contact. Wash off any splashes immediately.
2 Phenol should kill microorganisms present, but will not activate enzymes in the plant tissue. Phenol is described on the CLEAPSS Hazcard as TOXIC by inhalation, in contact with skin and if swallowed. There is possible risk of irreversible effects as it is a category 3 mutagen and causes burns. The technician making up the solution must wear goggles and disposable nitrile gloves, and refer to the Hazcard for details of what to do if the solid compound is spilled. The 1 % solution should be handled with care, but is much less hazardous than the solid compound.
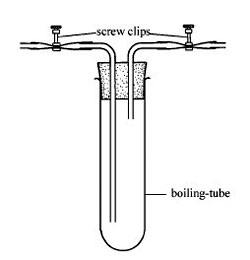
3 This apparatus shows a boiling tube into which you can introduce different atmospheres to see their effect on a slice of apple or potato. You can introduce gases via the input tube from a gas generator.
Generate carbon dioxide by adding dilute hydrochloric acid to marble chips (calcium carbonate) in a conical flask via a thistle funnel. Add about 50 cm3 of 0.1 M acid to a 250 cm3 beaker containing marble chips. The chips will effervesce for a while as the carbon dioxide is released. See CLEAPSS Hazcard: hydrochloric acid is an IRRITANT at concentrations about 2.0 M but is LOW HAZARD at this concentration.
Absorb oxygen from the air in a large conical flask using alkaline pyrogallol (see CLEAPSS Recipe card). Benzene–1,2,3-triol (pyrogallol) is HARMFUL (see CLEAPSS Hazcard). Wear eye protection when preparing the solution. 1 g of benzene-1,2,3-triol is capable of absorbing 190 cm3 of oxygen, but it does not absorb oxygen quickly. Prepare a saturated solution of sodium hydrogencarbonate using freshly-boiled pure water. Put about 100 cm3 of this solution in a 500 cm3 conical flask. Add a little more than 1 g of benzene-1,2,3-triol crystals to the flask. Stopper the container and leave overnight to absorb the oxygen. Displace the deoxygenated air into the apparatus illustrated above by adding water to the flask.
Alternatively, you could add carbon dioxide or nitrogen from pressurised gas cylinders.
4 Hydrochloric acid at this concentration is described on the CLEAPSS Hazcard as an IRRITANT. Wear eye protection when handling.
5 Citric acid (2-hydroxypropane-1,2,3-tricarboxylic acid) is described on the CLEAPSS Hazcard as an IRRITANT. Wear eye protection when preparing the solution.
6 Sodium hydrogensulfate(IV) (sodium hydrogensulfite) is described on the CLEAPSS Hazcard as HARMFUL. Solutions less than 0.25 M are LOW HAZARD. However sulfur dioxide gas is given off, which is TOXIC. It is dangerous if mixed with acids. Inhalation of sulfur dioxide by pupils or staff with known breathing difficulties such as asthma may exacerbate these pre-existing conditions. Teachers should be aware of staff and pupils with breathing problems, because the smell of sulfur dioxide can be detected from sulfite solutions.
7 Sodium chloride and sucrose are low hazard
Procedure
SAFETY:
Initial observation
a Cut an apple or potato into eight pieces. Take one piece and crush it in the mortar and pestle. Put the crushed sample on a tile next to an uncrushed piece. Compare the rate at which both samples go brown.
b When the uncrushed piece is brown, cut a small piece from it; also break a small piece from it. Note the colour of the freshly-exposed surfaces.
c Note which browns more quickly – the cut or broken surface?
Investigation 1 Is the reaction an effect of microorganisms acting on the tissue?
d Take two slices of apple or potato.
e Soak one in a 1% solution of phenol for 1 minute.
f Soak a similar slice in pure water as a control.
g Use tongs to remove the slices, shake off excess liquids Wash one surface of each with 3-4 drops of benzene-1,2-diol solution. Observe the rate of browning of each slice.
Investigation 2 Do enzymes control the reaction?
h Take four slices of apple or potato. Treat as described below:
- Immerse in a water bath at 100 °C for 1 minute.
- Immerse in a water bath at 60 °C for 1 minute.
- Immerse in a water bath at 40 °C for 1 minute.
- Immerse in water at room temperature for 1 minute.
i Remove the slices using tongs, and shake off excess liquid. Wash the surfaces with 3-4 drops of benzene-1,2-diol.
j Compare the rates at which the four samples go brown.
Investigation 3 Does the reaction require air or some part of the air?
k Put four slices of apple or potato in four separate boiling tubes.
l Wash the upper surfaces with 3-4 drops of 1% benzene-1,2-diol.
m Use the apparatus in note 3 to hold the apple or potato slices in different atmospheres, as described below:
- aatmosphere of nitrogen
- atmosphere of carbon dioxide
- normal atmospheric air
n Note the rate at which each slice goes brown.
Investigation 4 How does ascorbic acid affect the browning reaction?
o Take six slices of apple or potato.
p Soak each slice in one of the following solutions for 2 minutes:
- 5% ascorbic acid
- 3.5% ascorbic acid
- 2.5% ascorbic acid
- 2% ascorbic acid
- 1% ascorbic acid
- distilled water
q Remove the slices, shake off excess solution, and wash the upper surfaces with 3-4 drops of 1% benzene-1,2-diol.
r Arrange the tubes in sequence to show which goes brown first and which last.
Investigation 5 How do other chemicals affect the browning reaction?
s Take seven slices of apple or potato.
t Soak one in each of the following solutions for 2 minutes:
- 2% hydrochloric acid
- 2% citric acid
- 2% sodium hydrogensulfate(IV)
- 2% sodium chloride
- 2% sucrose
- boiling water
- cold distilled water
u Remove the slices, shake off excess solution, and wash the upper surfaces with 3-4 drops of 1% benzene-1,2-diol.
v Note how soon each slice goes brown.
Teaching notes
The observation that fruits and some vegetables discolour – usually turning brown – when we cut them is familiar. These investigations allow students to explore what causes the browning, and how to stop it. The control of fruit browning is not just of academic interest; it is of commercial importance in the processing of various fruit products. Although we generally find discoloured fruit or vegetables less appealing than those with ‘fresh’ colours, the browning reactions do not involve hazardous microbiological decay and so are safe to investigate in the laboratory.
Some of the methods of stopping fruit discolouration are hard to explain. The reaction is an oxidation, but is catalysed by an enzyme. Some methods or chemicals preventing browning act by preventing oxidation, but how does adding sugar prevent browning, or adding salt?
The reaction taking place in the first stage of browning is an enzyme-catalysed oxidation of benzene-1,2-diol to a quinone. In the second stage, the quinone reacts with water to form benzene-1,2,4-triol. Benzene-1,2,4-triol then reacts with any unchanged quinone, followed by a rearrangement to form a dicyclic compound. This then polymerises further to give highly-coloured pigments of unknown structure.
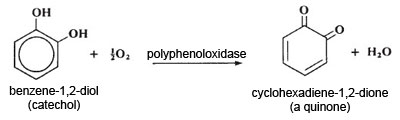
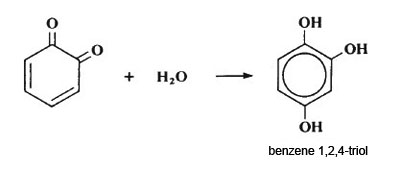
Ascorbic acid
The investigation with ascorbic acid is a qualitative one. If the students place the samples in order of increased delay period, they will see that delay period increases with increasing concentration of ascorbic acid.
Ascorbic acid is a reducing agent. It acts by reducing the quinone back to the hydroquinone. It is itself oxidised in the process. No pigment forms until all the ascorbic acid is oxidised. The enzyme activity slowly drops as it catalyses the reaction. If there is enough ascorbic acid, the enzyme loses its activity before all the ascorbic acid is oxidised and no discolouration occurs. Ascorbic acid is known as vitamin C and is widely distributed in nature, controlling the redox systems in living cells. It is a micronutrient and so is a very useful antioxidant in the food industry.
Other chemicals
Enzyme activity is affected by pH – with an optimum between pH 6.0 and 8.0 – and is almost completely inhibited below pH 3.0. The normal pH of apples is between 3.0 and 4.0, and that of potatoes is between 5.0 and 6.0 depending on ripeness, variety and time of year.
Adding acids reduces browning. Citric acid and malic acid occur naturally in fruits and so can be added safely to processed fruit without significantly affecting flavour. Hydrochloric acid will reduce browning, but cannot be safely added to food.
Sulfur dioxide is released from sodium hydrogensulfate(IV) and is commonly used as a preservative in food. It is a reducing agent and acts by preventing colour formation. Some people report a sensitivity to this chemical as a preservative in their foods.
Sodium chloride inhibits the enzyme by raising the ionic concentration.
Sucrose acts by reducing the solubility of oxygen in the surface tissues.
Health & Safety checked, May 2009
Downloads
Download the student sheet ![]() Investigating what makes fruit go brown (106 KB) with questions and answers
Investigating what makes fruit go brown (106 KB) with questions and answers
Web links
www.saps.org.uk/students/projects/171-student-project-catechol-oxidase-activity-in-fruits-and-vegetables
This practical on the SAPS website relates to the fruit browning reaction and involves extraction of the enzyme (catechol oxidase) from bananas and investigating how the enzyme action can be inhibited by lead. It might make an interesting extension in this context – considering why lead is not used to preserve the natural colours of food.
(Website accessed October 2011)


