Measuring respiratory quotient
Class practical
 This investigation involves handling whole living organisms and provides a quantitative method of exploring metabolism. Measurements of gas exchange can be made with a respirometer – not to be confused with a spirometer.
This investigation involves handling whole living organisms and provides a quantitative method of exploring metabolism. Measurements of gas exchange can be made with a respirometer – not to be confused with a spirometer.
This is done by measuring the change in the volume of gas surrounding the material as it respires – first as carbon dioxide is absorbed (to measure the rate of oxygen consumption) and then without absorbing the carbon dioxide (from which you can calculate the rate of production of carbon dioxide by comparison with the first measurment).
It is essential to eliminate volume changes that could be caused by anything other than the exchange of carbon dioxide and oxygen. The apparatus consists of two vessels (see diagrams and Note 1 below). One vessel contains the organisms and the other acts as a thermobarometer. Small changes in temperature or pressure cause air in this vessel to expand or contract, compensating for similar changes in the first vessel. Changes in the manometer level are thus due only to the activities of the respiring material.
Lesson organisation
This will depend on how many different living materials you wish to investigate. If your laboratory temperature is kept even, you may not need a water bath to maintain constant and equal temperatures for the two vessels. The suggested timing is a recording period of 30 minutes with carbon dioxide absorbed (to measure oxygen volume taken in) and again without carbon dioxide absorbed (from which carbon dioxide given out will be calculated). This requires a long working time for the practical. The respirometers are relatively small and fiddly to handle, so it is better if students working in pairs than threes with this apparatus.
Apparatus and Chemicals
For each group of students:
Respirometer, 1 (Note 1)
This is comprised of: two boiling tubes, one basket or cage (metal or plastic), one 1 cm3 syringe, two bungs (1-holed if using 3-way taps, 2-holed if these are not available), and one manometer (a capillary U-tube fixed to a scale on a board and filled with coloured oil – Note 2)
Potassium hydroxide solution, 15% (= 2.7 M), about 15 cm3 (Note 3)
Funnel
Filter paper, cut into strips, to form tight rolls
A small quantity (about 5 cm3) of living material such as seeds (germinating), woodlice, Calliphora larvae or a locust
Health & Safety and Technical notes
Take care with potassium hydroxide solution or any other carbon dioxide-absorbing materials. They are corrosive, so wear eye protection and wash any splashes off the skin immediately.
1 Respirometer: CLEAPSS Laboratory Handbook section 15.10 includes details of how to construct your own respirometers if you do not wish to purchase ready-made kit. Silicone rubber tubing is recommended, or aquarium pump tubing to connect to a 1 cm3 syringe. Short, narrow bits of tubing minimize wild movements of the manometer fluid caused by handling the apparatus. Rubber bungs are essential (not cork) as it is absolutely necessary that all joints are airtight. It might prove difficult to attach existing manometers and syringes to 3-way taps, in which case the 2-holed bung arrangement would be easier to set up.
When assembling a respirometer, push the connections together firmly but gently to achieve an airtight joint with low risk of breaking any glass tubing.
Make the respirometer airtight only after introducing the living material.
To check that the apparatus is airtight, move the marker fluid in the manometer to one end with the syringe and leave for a few minutes. The fluid should not move.
2 CLEAPSS Recipe card 13A describes several ways of making a suitable manometric fluid. Water strongly adheres to glass so an emulsifier is needed to prevent water droplets “sticking” to the walls of the manometer and so affecting the readings, and to reduce capillary action in small-bore manometers. The simplest version is water containing a food dye such as cochineal with a few drops of detergent.
3 Potassium hydroxide: Hazcard 91 describes this chemical as corrosive. Wear goggles when handling. If any of the solution gets into the eyes, flood the eye with gently-running tap water until a first-aider arrives. Send the affected person to hospital and ensure that irrigation is continued during the journey.
It can be very difficult to remove the living material from the tube without contaminating it (and so damaging it) with the hydroxide solution.
4 It is best to use oil-bearing seeds for this investigation. Avoid castor-oil seeds as they are toxic if eaten. Sunflower seeds are a suitably low hazard option. If seeds containing carbohydrate food reserves are used, the second part of the investigation will be uneventful: there will be little or no change in net volume. Although this is still instructive, it may not feel rewarding.
Ethical issues
There are no ethical issues if using plant material for this investigation. If using invertebrates (such as woodlice, Calliphora larvae or locusts) it is important to handle the organisms with care and respect, particularly ensuring that they do not come into contact with the potassium hydroxide solution. Teachers should be careful to introduce any animals used in a way that promotes a good ethical attitude towards them and not simply an instrumental approach. Although they are simple organisms that may not 'suffer' in the same way as higher animals, they still deserve respect. Return the animals promptly to their holding tank or natural environment after the investigation. This supports ethical approaches that are appropriate to all field work where animals are returned to their habitat after observations have been made.
Procedure
SAFETY: Take care with potassium hydroxide which is corrosive. Wear goggles and wash any splashes off the skin immediately.
Assemble the apparatus by pushing the tubes together firmly but gently. If you force sections of apparatus together, you risk breaking glass components and cutting yourself.
Preparation
a Use a funnel to pour 5 cm3 of potassium hydroxide solution (corrosive) into each respirometer vessel. Make sure none of the potassium hydroxide touches the sides of the vessels.
b Add small rolls of filter paper to act as wicks.
c Fill the basket or cage with respiring material (Note 4) and put it into vessel B. Make sure that the seeds or invertebrates are not touching the potassium hydroxide or the wick. Add water to vessel A to match the volume of respiring material in vessel B – see diagram.
d Fit vessel A with a bung holding two connecting tubes – one with a screwclip on a flexible hose. Alternatively fit a bung with a 3-way tap connected to the same items.
e Fit vessel B with a bung holding a 1 cm3 syringe and a connecting tube as shown in the diagram. Alternatively fit a bung with a 3-way tap connected to the syringe and tube.
f Draw some coloured fluid into the manometer U-tube. The fluid must be free of bubbles and come to about the middle of the scale on each side.
g Open the screw clip and remove the syringe, then connect the manometer U-tube. To check that the apparatus is airtight, move the marker fluid in the manometer to one end with the syringe and leave for a few minutes. The fluid should not move.
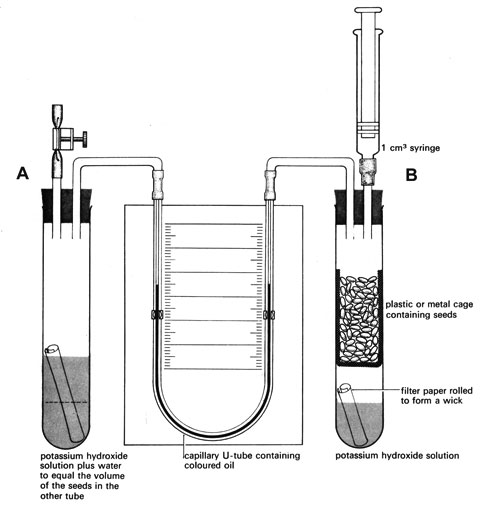
h Set the piston of the syringe at about the 0.5 cm3 mark and insert the syringe as shown. Close the screw clip. Use the syringe to adjust the manometer so that the fluid levels are the same on both sides.
i Record the exact position of the syringe piston, the position of the menisci on both sides of the manometer, and the time.
Investigation
j Record new positions of the manometer fluid at regular intervals for 30 minutes. When it nears the end of the scale on one side, restore it to its original position and note the new position of the syringe piston.
k Find the amount of oxygen absorbed by germinating seeds in a period of 30 minutes at 20 °C. This is Vol1.
l Remove the potassium hydroxide solution from both vessels and wash them out with water.
m Replace the basket containing seeds or invertebrates in vessel B, an equivalent volume of water in the other vessel and the bungs in both. Set up the respirometer a 20 °C again and record any increase or decrease in gas volume over the next 30 minutes. This is Vol2.
n Calculate the volume of carbon dioxide produced.
o Calculate the respiratory quotient.
Teaching notes
The volume of oxygen absorbed is Vol1. This is recorded in the first part of the investigation.
Vol2 is the volume of carbon dioxide produced minus the volume of oxygen absorbed. This is recorded in the second part of the investigation.
Therefore, Vol1 + Vol2 = the total volume of carbon dioxide produced.
Respiratory Quotent = Volume of carbon dioxide produced
___________________________
Volume of oxygen absorbed

RQ must be interpreted with care and, on occasion, may not really tell us very much. If an organism is respiring a mixture of compounds, the RQ that results will be the weighted average of the RQs of the mixture and will depend on the proportions of each substance involved.
Theoretical values for the respiratory quotient can be calculated from balanced equations for the complete oxidation of compounds. Examples are given here for glucose (C6H12O6) and tristearin (C57H110O6 – a fat).
C6H12O6 + 6 O2 ? 6 H2O + 6 CO2
2 C57H110O6 + 163 O2 ? 110 H2O + 114 CO2
| Substrate | Oxygen absorbed | Carbon dioxide released | RQ |
| glucose | 6 | 6 | 1.00 |
| tristearin | 163 | 114 | 0.69 |
RQ becomes less meaningful if the living material is respiring anaerobically. If little or no oxygen is taken in, the RQ tends to infinity. Many germinating seeds are known to respire anaerobically and so their RQs will depend on the type of respiration as well as the substrates respired. The greater the fraction of anaerobic respiration, the larger the RQ will become.
Downloads
Download the student sheet ![]() Measuring respiratory quotient (0.9 MB) with questions and answers.
Measuring respiratory quotient (0.9 MB) with questions and answers.
Related experiments
Measuring the rate of metabolism
This practical introduces the use of the respirometer and uses oxygen uptake simply to assess the rate of metabolism.


