Working with immobilised enzymes or microscopic organisms
Class practical
Enzymes are essential catalysts for the chemical processes of life. We exploit the properties of enzymes in research and industry, using them to catalyse chemical reactions or to synthesize products. Sometimes we catalyse a reaction by using entire microscopic organisms (algae, yeast or bacteria) rather than a purified enzyme.
Immobilising enzymes or microscopic organisms involves trapping them in a matrix of an inert material or binding them to its surface. This makes it easier to remove the active catalysts from the reaction mixture, and so makes it easier to purify the products. It also allows us to set up systems for continuous processing, packing the immobilised catalysts in a vessel through which a steady stream of reactants can flow – collecting useful products at the outlet.
This procedure describes how to make alginate beads containing yeast. These are used to catalyse the fermentation of glucose to ethanol. The method can be applied to other catalysts in other reactions, or to more sophisticated investigations of enzyme action. See links below.
Lesson organisation
Students will probably find making alginate beads quite entertaining, at least the first time. Allow each group to make some beads. Set up a flask containing free yeast as a demonstration. Each group could either set up the beads in a conical flask with glucose solution, or pack a column with beads and try to set glucose flowing through suitably slowly.
Apparatus and Chemicals
For each group of students:
Clamp stand, 1
Boss and clamp, 1
Syringe barrel, 1
Pasteur pipette tip, 1 (Note 4)
Other reagents and glassware relevant to the specific practical to be followed (see teaching notes).
For the class – set up by technician/ teacher:
Calcium chloride solution, 1.5%, 50 cm3 per group (Note 2)
Sodium alginate solution, 4% about 100 cm3
Yeast suspension 10%, about 100 cm3
(Note 3)
Glucose solution, 8%, 200 cm3 per group
Health & Safety and Technical notes
Avoid skin contact with enzymes in solution and with calcium chloride solution.
Avoid exposure to enzyme as dry powder – by using a fume cupboard if preparing enzyme solutions, or by wiping up spills of enzyme solutions so they do not dry out.
1 Preparing solutions to make alginate beads (See CLEAPSS Recipe card): Dissolve sodium alginate in 100 cm3 of cold, distilled or deionised water. Warm gently if it does not dissolve readily, or start again with warm water. Stir with a spatula every half hour or so. Leave overnight and stir again in the morning. There are variations in alginate from different suppliers and in different batches. It is worth carrying out a trial run, and adjusting the concentration of alginate if necessary. If the alginate gel is too dense, the substrate cannot enter the bead. Use 4% alginate to mix with equal volumes of yeast suspension. Use 2% alginate to mix 4:1 with enzyme solution.
2 Calcium chloride solution: Dissolve 4 g of calcium chloride-2-water in 200 cm3 of pure water in a 250 cm3 beaker. The CLEAPSS Hazcard describes calcium chloride as an IRRITANT, so wear eye protection when making up solution. Once made, this 4% solution is LOW HAZARD. Anhydrous calcium chloride (only 3 g in 200 cm3) could be used, but take care as it could make the water boil. The calcium ions need to be in excess for this to work well. They form cross-links of calcium alginate in the gel, trapping the enzyme or yeast within the matrix.
3 Enzymes: the CLEAPSS Hazcard suggests that many enzymes are harmful as powders. Solutions at a concentration of 1% and above are an IRRITANT, but below 1% enzyme solutions are LOW HAZARD.
4 If your Pasteur pipette tip produces beads that are too big, use instead a drawn-out glass capillary tube, attached to the syringe with about 2 cm of flexible rubber or plastic tubing.
Procedure
SAFETY: Take care handling the calcium chloride. Do not consume any of the products of a reaction carried out in a laboratory where chemical or biological contamination could occur, or using non-food-grade reagents, or apparatus that has been used for other chemicals or biological materials.
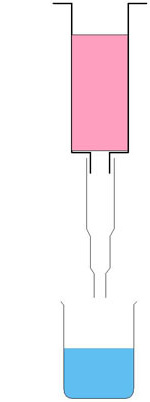 Preparation
Preparation
a Make up the alginate solution and the calcium chloride solution. (See Notes 1 and 2.)
b As close to the time of use as possible, make up the yeast suspension or enzyme solution with distilled water.
c Set up the apparatus as shown in the diagram to the right. Put calcium chloride solution in the beaker. A Pasteur pipette tip is attached to a syringe supported in a clamp, so that the tip of the pipette is 5 cm above the surface of the calcium chloride.
Investigation
d Mix equal volumes of alginate solution and yeast suspension to give a final concentration of 2% alginate. (Note 1.)
e Pour the mixture into the syringe barrel and allow it to drip through into the calcium chloride solution as in the diagram above.
f Separate the alginate beads from the calcium chloride solution using a small sieve or tea-strainer and rinse with a little distilled water.
g Use the beads as a source of catalytic enzyme in a batch fermentation process. Alternatively, pack the beads into a column and run the solution of reactants slowly past the beads to demonstrate a continuous reaction process. You can use a syringe barrel or a longer burette to make a column. A useful tip is to place some nylon mesh in the bottom of the syringe barrel; otherwise the alginate beads will block the outlet.
h Collect the products of the reaction.
Teaching notes
You could run this practical in a variety of ways.
Set up batch fermentation of glucose with yeast using free yeast in some vessels and immobilised yeast in others
Quantify the rate of fermentation using a bubble logger as described by NCBE. After a few days, separate the yeast from the reactants and products – by filtration, centrifugation, or simply straining out the beads.
Compare the ease of removal of yeast in each process. Evaluate the process in terms of how long it takes, how clear the final mixture is, and how easy it would be to use the yeast again.
Quantifying the success of the reaction in terms of production: extract the ethanol by distillation to estimate yield, or compare the specific gravity (density) of the resulting solution with that of 8% glucose to estimate yield. Density should change from 1008 g per litre to 1004 g per litre. The hydrometer from a home brewing/ wine-making kit would be most appropriate for measuring this quantity.
Co-entrap brewer’s yeast (Saccharomyces cerevisiae) and the enzyme ? galactosidase (lactase)
The yeast can ferment sugars such as glucose and sucrose to produce ethanol and carbon dioxide, but it cannot usually ferment lactose. If it is entrapped with lactase, it will be able to ferment the sugars produced by the action of the lactase.
Compare the rate of fermentation of lactose by yeast immobilised in alginate beads with and without lactase. More details of this NCBE procedure.
Immobilise an enzyme to change one chemical into another
Milk contains significant quantities of the carbohydrate lactose, a disaccharide made from linked glucose and galactose. Adult humans and cats often like drinking milk, but many of them do not produce enough active lactase in their digestive systems to process the lactose. Non-digestion or mal-digestion of lactose can cause digestive upsets.
Treating milk with the enzyme lactase removes the lactose and makes the carbohydrates digestible. This can be done by adding lactose to milk or by running the milk through a column containing immobilised lactose and collecting the 'improved' (lactose-reduced) milk at the outlet.
In this case, use 8 cm3 of 2% sodium alginate solution, mixed with 2 cm3 of lactase (?-galactosidase) such as Novozymes lactozym® from NCBE. More details of this NCBE procedure.
Health & Safety checked, May 2009
Web links
www.ncbe.reading.ac.uk
The National Centre for Biotechnology Education has a huge number of protocols for practical biotechnology activities and some associated biology practicals. They also supply enzymes and consumables for biotechnology practical work.
There are several useful practicals on fermentation downloadable from the NCBE website at www.ncbe.reading.ac.uk/NCBE/PROTOCOLS/fermentation.html. This booklet includes details of logging bubble rate.
NCBE protocol for immobilised yeast: http://www.eurovolvox.org/Protocols/immobilisedyeast.html
NCBE protocol for immobilised ?-galactosidase: http://www.eurovolvox.org/Protocols/catmilk.html
http://www.nationaldairycouncil.org/Pages/Home.aspx
Information about lactose intolerance and the nutritional value of dairy foods.
www.lsbu.ac.uk/biology/enztech/
London South Bank University site with more information about the use of immobilised enzymes in industry. Includes Enzyme technology by Martin Chaplin and Christopher Bucke (Cambridge University Press, 1990). The book is out of print, but the text is available here. 'Methods of immobilization' in Chapter 3 includes diagrams of the different ways an inert matrix can contain or simply bind an enzyme. Chapter 5 includes more technical information about the design of enzyme reactors and examples of enzymes used in industry – particularly the food industry.
(Websites accessed October 2011)


