How much energy is there in food?
Class practical
Take samples of a range of foodstuffs and set them alight in turn. Burn food samples under a boiling tube containing a measured amount of water. Measure the temperature increase in the water. Calculate the amount of energy needed to cause that temperature increase. This gives an estimate of the amount of energy stored in the food.
The apparatus is very simple and the protocol gives only a very approximate estimate for each foodstuff, so there is scope for students to suggest improvements in an evaluation.
Lesson organisation
This could be a teacher demonstration, or carried out by students in groups of two or three.
Apparatus and Chemicals
For each group of students
Boiling tube, 1
Clamp stand, boss and clamp, 1
Bunsen burner, 1 at each station
Heatproof mat, 1
Measuring cylinder, 50 cm3 or 100 cm3, 1
Mounted needle with wooden handle, 1 (Note 2)
Tongs or forceps for food samples that cannot be impaled
Thermometer (–10 °C–110 °C)
Eye protection
Water
For the class – set up by technician/ teacher:
Food samples, range of foods in small pieces (cut to approximately 1 cm square/ 0.5 cm cubed if necessary) – for example, cheese, pulses, bread, biscuits, pasta, packet snacks such as crisps and others, breakfast cereals. Nuts are best avoided
(Note 1).
Balance, accurate to ± 0.1 g
Health & Safety and Technical notes
Students will be working with small flames and hot equipment.
Some students may be allergic to some of the foodstuffs or the fumes produced by burning them. Be alert to the signs of allergic reactions in your students – such as skin rashes or breathing difficulties – be prepared to administer first aid.
The risk of anaphylactic shock following allergic reaction to peanuts (or other nuts) is such that it is probably best to avoid nuts as foods for this investigation (Note 2).
1 Food allergies: Avoid nuts as foods for this investigation. Check with the students if they have any known allergies and ask them to avoid the foods to which they are sensitive. Be aware of your first aid procedures in case of extreme reactions. See CLEAPSS Laboratory Handbook, General Equipment section 9.4.2 for detailed precautions to take if you are determined to use peanuts. A well-ventilated laboratory is not considered adequate.
2 Avoid mounted needles with metal handles for this practical as the handles could get hot.
3 As students impale the food on the mounted needle, make sure they do not stick the needle into their hands.
4 Fatty foods especially burn with a very smoky flame. Students can reuse the same boiling tube repeatedly in this practical, but the washing up might be quite difficult.
5 The CLEAPSS Laboratory Handbook has instructions for making a wire-gauze basket for foods that do not ignite or burn easily. You can line one of these with Superwool 607 and to hold liquid foods and foods that melt on heating.
Ethical issues
Discussing the energy content of food can lead to discussion of body mass and weight loss which is a sensitive area for many students.
Procedure
SAFETY: Check in advance if any students have known food allergies. Ensure none of those allergens are used for the procedure by any group.
Be prepared with first aid for minor burns.
Check all students are wearing eye protection during the procedure.
Warn students to avoid foods to which they might have allergies.
Take care with mounted needles.
Preparation
a Dry samples of foods that will burn better if dried – for example, olives dried for 3 days at 50°C, cubes of cheddar cheese or mini marshmallows dried on a paper towel on a windowsill for a few days. Make sure food drying like this does not attract mice or other vermin.
b Cut up a range of foods into small pieces – around 1 cm square or 0.5 cm cubed.
Investigation

a Use the measuring cylinder to measure 20 cm3 of water into the boiling tube.
b Clamp the boiling tube to the clampstand.
c Measure the temperature of the water with the thermometer. Record the temperature in a suitable results table.
d Choose a piece of food and find its mass using the balance. Record the mass in the table.
e Impale the piece of food carefully on a mounted needle. (Note 3)
f Light the Bunsen burner and hold the food in the flame until it catches alight.

g As soon as the food is alight, put it under the boiling tube of water as shown. Try to make sure that as much of the heat from the burning food as possible is transferred to the water. Do this by keeping the flame under the tube.
h Hold the food in place until the food has burnt completely. If the flame goes out, but the food is not completely burnt, quickly light it again using the Bunsen burner and replace the food beneath the tube.
i As soon as the food has burned away completely and the flame has gone out, measure the temperature of the water again. Before measuring, stir the water carefully with the thermometer and note down the highest temperature reached in the results table.
j Repeat the procedure for other foods. (Note 5)
k Calculate the rise in temperature each time.
l Calculate the energy released from each food by using this formula.
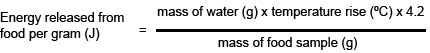
4.2 is the value of the specific heat capacity of water, in joules per gram per degree Celsius – the number of joules taken to raise the temperature of water by 1 °C. 1 cm3 of water has a mass of 1 g.
If the number is more than 1000 J/g, express it as kilojoules (kJ):
1 kilojoule = 1000 joules
m Compare your results for each food with the rest of the class.
Teaching notes
Make a note of the energy values for the foods used from their packaging. It is usually quoted for 100 g of the food. Compare this value with the estimates from this activity.
Although joules are the SI units for energy, you might want to talk in terms of calories when discussing food. Most students will be more familiar with thinking about calorie content than joule content of foods! A calorie is the amount of energy needed to raise the temperature of 1 cm3 (or 1 g) of water by 1 °C. So you can use the formula above, but take out the 4.2 for the specific heat capacity of water. Most students believe that a healthy diet is between 1000 and 2000 ‘calories’ per day, and so they are surprised how many calories are estimated in a single peanut by this experiment. This is a good place to point out that what we commonly call ‘calories’ in food are really kilocalories (kcal on the food packaging), 1 kilocalorie = 1000 calories. This is a useful example of a situation where accurate use of a scientific term seems to contradict normal use of the term in our language!
This apparatus is very simple and there are many ways in which it could be improved. For example, using a soft drinks can as a draught shield, using more or less water, considering whether a test tube is better than a boiling tube. With some foodstuffs, small volumes of water will boil.
The student sheet shows an alternative apparatus for collecting the heat from burning food. You will probably find several variations on this in your textbooks. Have a look in the prep room for one, too. Comparing and contrasting the features of each, students could develop their own designs for calorimeters.
Health and safety checked, September 2008
Downloads
Download the student sheet ![]() How much energy is there in food? (145 KB) with questions and answers.
How much energy is there in food? (145 KB) with questions and answers.
Web links
http://www.nhs.uk/LiveWell/Goodfood/Pages/Goodfoodhome.aspx
This NHS website has information for ‘Teens’ area about dietary recommendations as well as quizzes to self-check knowledge of and attitudes to food.
(Website accessed October 2011)


