Microscale investigations of catalase activity in plant extracts
Class practical
This protocol is adapted with permission from information on the Science and Plants for Schools (SAPS) website (see www.saps.org.uk). Hydrogen peroxide (H2O2) is a by-product of respiration and is made in all living cells. Hydrogen peroxide is harmful and must be removed as soon as it is produced in the cell. Cells make the enzyme catalase to remove hydrogen peroxide. Different plant materials show very different amounts of catalase activity – and the most metabolically active tissues show the greatest activity. The procedure can be used to show differences in activity in imbibed and germinating seeds and in damaged or decaying plant material.
Lesson organisation
Producing the cell extracts may take 20-30 minutes for the class depending on the number of microcentrifuges you have available. Assessing the catalase activity of each sample will take 5-10 minutes depending on number of repeats. Students working in small groups (2-4) to follow their own investigation should each find a role that keeps them busy.
Apparatus and Chemicals
For each group of students:
Microcentrifuge tubes, 2 per plant sample
Mortar and pestle
Silver sand
Filter paper, cut into discs with a hole punch, up to 10 per sample
Filter paper, 1, to blot disc samples
Comboplate®, 1 per working group (Note 4)
Specimen tubes, small, 1 per working group (if preferred to Comboplate®)
For the class – set up by technician/ teacher:
Microcentrifuge or other centrifuge (Note 1)
1% hydrogen peroxide, refer to Hazcard 50 (Note 2)
pH 7 buffer, about 1 cm3 per plant sample
(Note 3)
Health & Safety and Technical notes
Take care when making up the dilute solution of hydrogen peroxide (refer to Hazcard 50 for more information).
1 The method is quick with a microcentrifuge, but if you don’t have one, you can use a normal centrifuge but you will need larger amounts of plant material.
2 The volume of 1% hydrogen peroxide needed depends on the reaction vessel – about 5 cm3 in a specimen tube, or 2 cm3 in a large Comboplate® well. If reaction times are longer than 2 minutes, use a more concentrated hydrogen peroxide solution. If reaction times are less than 5 seconds, use a less concentrated solution. At this concentration (approximately 3 vol) hydrogen peroxide is described as low hazard (see CLEAPSS Hazcard), but take appropriate precautions in preparing the dilute solution – store in brown bottles, do not dilute more than you need, and use as quickly as possible.
3 Add only the minimum amount of buffer to make the extract. Never use a greater mass of buffer than of plant matter. With a watery tissue, you may not need to add any buffer. You can sometimes collect the cell contents by holding the dry disc of filter paper in contact with the freshly cut surface of the fruit or vegetable.
4 Comboplates® are plastic microplates with numerous wells in two sizes. They are stable and provide a series of reaction vessels which makes them ideal for this procedure. They are available from EDU-LAB in Norfolk (see Suppliers).
5 When carrying out repeats, there is no need to change the hydrogen peroxide solution until you have used more than 10 discs.
Ethical issues
There are no ethical issues associated with this procedure.
Procedure
SAFETY: Take care in handling hydrogen peroxide as described on the CLEAPSS Hazcard.
Preparation
a Make up just enough hydrogen peroxide solution, close to the time when you will need to use it (Note 2).
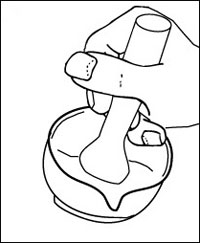
b Grind the plant material in a mortar with a small amount of pH 7 buffer and silver sand (Note 3).
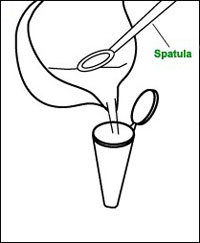
c Scrape the paste into a microcentrifuge tube and spin at high speed until a pellet forms (Note 1).
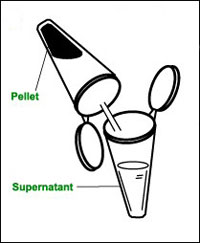
d Transfer the supernatant to a clean labelled microcentrifuge tube. Store the tube in a beaker of crushed ice if you cannot use it immediately, then bring to room temperature before use. The extract will not keep overnight, even in a fridge.
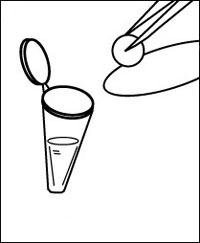
e Dip a dry disc of filter paper in the extract then blot briefly on a sheet of filter paper.
Investigation
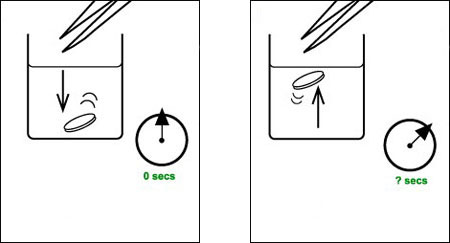
f Set up your reaction vessel with at least 1 cm depth of 1% hydrogen peroxide solution in a position where you can get a clear view of the vessel from the side. A depth of less than 2 cm gives more precise timing.
g Drop in the disc so that it settles on the bottom, and start timing. If the disc settles at the side, or gets caught on the wall of the vessel, remove it and repeat the process.
h Note the time taken for the disc to rise to the surface of the hydrogen peroxide solution, then remove the paper disc.
i Repeat stages f and h to get reliable timings (Note 4).
Teaching notes
Suggested investigations include:
- establishing the time-course of catalase activity in whole seeds during the germination process
- how do the following stresses affect the catalase levels of seedlings?
- flooding (for example, by extended periods of immersion in water)
- heat/ cold shock (give heat/ cold treatments to imbibed seeds for at least 1 hour)
- shortage of water
- how does catalase activity vary across or along the length of a larger vegetable or organ?
- how does catalase activity vary from a healthy to a diseased region?
This table shows fruits and vegetables with significant or absent catalase activity.
| Significant catalase activity | Catalase activity absent | |
| apricots | broccoii | apples |
| bananas | carrots | citrus fruit |
| cherries | cucumber | peaches |
| fresh flowers | parsnip | rhubarb |
| leeks | potato | tomato |
| onions | red cabbage | senescent flowers |
| turnips | ||
The advantage of using small samples of tissue is that you can take a series of samples across a plant organ (for example a diseased vegetable) and investigate the difference in catalase activity in different areas.
Oxygen may be trapped for longer in tissues with smaller air spaces.
Health & Safety checked, October 2008
Related experiments
Investigating the effect of concentration of blackcurrant squash on osmosis in chipped potatoes
Web links
http://www-saps.org.uk Search the SAPS website for their resources for working with enzymes.
This page http://www.saps.org.uk/secondary/teaching-resources/293-student-sheet-24-microscale-investigations-with-catalase contains details of this procedure and more background information on the biology of catalase in seeds.
(Website accessed October 2011)


