Investigating effect of temperature on the activity of lipase
Class practical
Phenolphthalein is an indicator that is pink in alkaline solutions of about pH10. When the pH drops below pH 8.3 phenolphthalein goes colourless. Here, an alkaline solution of milk, lipase and phenolphthalein will change from pink to colourless as the fat in milk is broken down to form fatty acids (and glycerol) thus reducing the pH to below 8.3. The time taken for this reaction to occur is affected by temperature.
Lesson organisation
This investigation could be carried out as a demonstration at two different temperatures, or in a group of at least 5 students with each student working at a different temperature. This would allow students to collect repeat data at their allocated temperature. Or it could be an investigation carried out by one student.
Apparatus and Chemicals
For each group of students:
Marker pen
Test tube rack
Measuring cylinder (or syringe), 10 cm3, 2
Beaker, 100 cm3, 2 (for milk and sodium carbonate solution)
Beaker, 250 cm3, 2 (to act as water baths for temperatures below room temperature)
For each temperature:
Thermometer
Test tube
Glass rod
Syringe, 2 cm3
Stop clock/stopwatch
For the class – set up by technician/ teacher:
Milk, full-fat or semi-skimmed, 5 cm3 per student per temperature assessed
Phenolphthalein in a dropper bottle (Note 2)
5% lipase solution, 1 cm3 per student per temperature assessed
Sodium carbonate solution, 0.05 mol dm–3, 7 cm3 per student per temperature assessed
Electric hot water baths set to a range of temperatures, each containing a thermometer, a test-tube rack and a beaker of lipase solution.
Ice
Health & Safety and Technical notes
Sodium carbonate solution, 0.05 M. Make with 5.2 g of anhydrous solid, or 14.2 g of washing soda per litre of water. See CLEAPSS Hazcard; it is an IRRITANT at concentrations over 1.8 M.
Ethanol (IDA) in the phenolphthalein indicator is described as HIGHLY FLAMMABLE on the CLEAPSS Hazcard (flash point 13 °C) and HARMFUL (because of presence of methanol).
Glassware is breakable.
Electric water baths should be safety checked in accordance with your employer’s instructions.
Take care with thermometers and brief students how to react if they are broken.
1 Lipase solution is best freshly made, but it will keep for a day or two in a refrigerator. Don’t try to study different temperatures on different days for the same investigation; the activity of the enzyme will change and it will not be a fair test.
2 Phenolphthalein is described as low hazard on CLEAPSS Hazcard. Refer to Recipe card (acid-base indicators): Dissolve 1 g in 600 cm3 of IDA then make up to 1 litre with water. Label the bottle highly flammable. Suppliers of phenolphthalein solution may not use IDA; it also may be diluted. Follow any hazard warning on supplier’s bottles.
Procedure
SAFETY: Keep the phenolphthalein solution away from sources of ignition.
Wear eye protection and quickly rinse any splashes of enzyme solution or sodium carbonate from the skin.
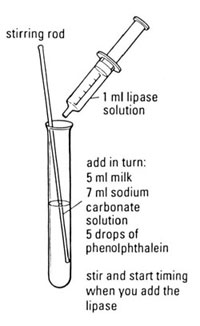 Preparation
Preparation
a Make up lipase solution and suitable quantities of the other solutions.
b Set up the water baths at a range of temperatures and put a beaker of lipase, containing a 2 cm3 syringe into each water bath. Cover a range of temperatures up to around 60°C. An ice-bath will maintain a temperature of 0°C, until all the ice is melted.
Investigation
c Label a test tube with the temperature to be investigated.
d Add 5 drops of phenolphthalein to the test tube.
e Measure out 5 cm3 of milk using a measuring cylinder (or syringe) and add this to the test tube.
f Measure out 7 cm3 of sodium carbonate solution using another measuring cylinder (or syringe) and add this to the test tube. The solution should be pink.
g Place a thermometer in the test tube. Take care as the equipment could topple over.
h Place the test tube in a water bath and leave until the contents reach the same temperature as the water bath.
i Remove the thermometer from the test tube and replace it with a glass rod.
j Use the 2 cm3 syringe to measure out 1 cm3 of lipase from the beaker in the water bath for the temperature you are investigating.
k Add the lipase to the test tube and start the stopclock/ stopwatch.
l Stir the contents of the test tube until the solution loses its pink colour.
m Stop the clock/ watch and note the time in a suitable table of results.
Teaching notes
The quantities used should take approximately 4 minutes to change from pink to white at normal laboratory temperature. If this is not the case, change the concentration of enzyme to alter the speed of the reaction (more enzyme will reduce the time or increase the speed). Students will need to use the same volume at each temperature.
Digestion of fat produces fatty acids (and glycerol) that neutralise the alkali, sodium carbonate, thus lowering the pH and changing phenolphthalein from pink to colourless. You could use a pH probe or data logger, or another indicator.
You could add washing-up liquid to the solution (1 or 2 drops per 250 cm3), to emulsify the fats which will provide a larger surface area for enzyme action. This will demonstrate the effect of bile salts. Or bile salts could be used.
Other factors to test:
- This protocol is based on a pH dependent result, so is not suitable for assessing the effect of different pHs on lipase.
- It would be possible to vary the concentration of the lipase and look at the effect of enzyme concentration on the breakdown of fat in milk.
- Different types of milk could be used Jersey, full cream, semi-skimmed and skimmed, to explore the effect on the reaction of changing fat concentration (substrate concentration).
Question 6 on the student question sheet opens the doors to a more extensive piece of research on this enzyme.
Health and safety checked, September 2008
Downloands
Download the student sheet ![]() Investigating effect of temperature on the activity of lipase (72 KB) with questions and answers.
Investigating effect of temperature on the activity of lipase (72 KB) with questions and answers.
Related experiments
Investigating an enzyme-controlled reaction: catalase and hydrogen peroxide concentration
Investigating effect of concentration on the activity of trypsin


