Investigating effect of concentration on the activity of trypsin
Class practical or demonstration
Exposed and developed black and white negative film is black because it contains light-sensitive silver halides (salts). Black and white film is composed of a plastic backing covered with a mixture of gelatine (protein) and silver halides. Digestion of the gelatine (protein) by trypsin releases the silver salts and the film clears. The time taken for the film to clear will be dependent on the concentration of trypsin used.
Lesson organisation
This experiment could be done as a demonstration at two or three different concentrations, or with a group of students each working at a different concentration. This would allow students to collect repeat data at their allocated concentrations. Or it could be an investigation carried out by one student.
Apparatus and Chemicals
For each group of students:
Wooden spills, 6 pieces
Test tubes, 6
Test tube rack, 1
Measuring cylinder, 10 cm3, 2
Beaker, 400-500 cm3, 1 (to act as water bath)
Thermometer (accurate to ± 1 °C around 35 °C)
Stopclock/ stopwatch
Distilled water
Hot water (tap water or a kettle)
Scissors (if students are to cut their own strips of film)
Marker pen
For the class – set up by technician/ teacher:
5% trypsin solution, 30 cm3 for each working group
35 mm photographic film, gelatine coated, cut into strips (Philip Harris supply exposed film in 1.5m strips)
Health & Safety and Technical notes
Trypsin solution at concentrations from 1% to 5% is an irritant. Wash splashes from the skin as quickly as possible. Wear eye protection. Ensure students inform you if any trypsin gets in their eyes. (Note 1.)
Silver halides in photographic film are low hazard (See CLEAPSS Hazcard).
1 Trypsin solution is best freshly made. (See CLEAPSS Hazcard and Recipe card). Add a pinch of sodium hydrogencarbonate to shift the pH towards the optimum for the enzyme. The powder is harmful; solutions from 1% to 5% are irritant. Enzymes can be kept in the refrigerator after they have been made up for use with different classes. However the activity declines with time, especially the activity of proteases, so the whole experiment should be completed in one session with each class.
2 Old-fashioned photographic film must be used; modern films do not necessarily use gelatine coatings.
Ethical issues
Gelatine is a by-product of the meat-processing industry, so you are working with an animal product. Be aware of students who may have religious or ethical objections to handling such material.
Procedure
SAFETY: Wear eye protection and quickly rinse any splashes of enzyme solution from the skin.
Preparation
a Make up 5% trypsin solution.
b Cut pieces of photographic film to fit into your standard test tubes. Using 35 mm stock, cut across the film to make strips 1 cm wide.
Investigation
c Label a test tube with the concentration of trypsin to be investigated.
d Use a measuring cylinder to measure volumes of 5% trypsin solution and distilled water as shown in the table below. Make up each concentration in a clean test tube.
| Concentration of trypsin in final solution (%) |
Volume of 5% trypsin needed to make 10 cm3of solution (cm3) |
Volume of water needed to make 10 cm3 of final solution (cm3) |
Final volume of trypsin solution made up (cm3) |
| 0 |
0 |
10 |
10 |
| 1 |
2 |
8 |
10 |
| 2 |
4 |
6 |
10 |
| 3 |
6 |
4 |
10 |
| 4 |
8 |
2 |
10 |
| 5 |
10 |
0 |
10 |
e Place the test tubes and the thermometer in a beaker of warm water at close to 35 °C. Maintain the temperature within two degrees of this during the course of the investigation by adding more hot water as necessary.
f Make a note of the temperature of the water.
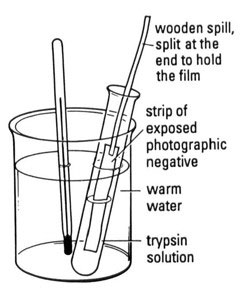
g Cut pieces of photographic film to fit in the test tube as shown. This may have been done for you.
h Attach wooden spills to each piece of film to be used (see diagram), or tie a piece of cotton thread through a sprocket hole. You could staple the film to the splints.
i Place the pieces of film in the test tubes as shown in the diagram and start the clock.
j As each piece of film clears, make a note of the time on the stopclock/ stopwatch. Do not turn off the stopclock/stopwatch.
k Plot a suitable graph of the results.
Teaching notes
The dependent variable in this investigation is time. You can calculate the rate of the reaction by calculating
1 ÷ time and draw a graph of rate of reaction against concentration of trypsin.
There may be a maximum rate of reaction in this investigation, but not an optimum – increasing concentration beyond the maximum should not reduce the rate of reaction.
The pH is not controlled in this experiment. The size of the pieces of film has also not been exactly specified. You could ask students if this is a fair test, and how it could be improved.
Measuring transmission through the film with a colorimeter could make the end-point more definite. Take care, not to drip the solution into the colorimeter – place the film in a cuvette or dry it first. However this introduces the risk of rubbing off the blackened silver halides. Depending on your cuvettes, you may be able to set up a strip of film in trypsin solution to react within the cuvette.
Students could be asked to design their own investigation of other factors such as:
- temperature – but at high temperatures the gelatine may simply melt which invalidates the result
- pH – using buffers to maintain a pH (see also Investigating the effect of pH on amylase)
- comparing the effects of trypsin with the effects of other enzymes – lipase or amylase – to demonstrate the specificity of enzyme action.
- the inhibitory effects of heavy metal ions.
Vegetarian and vegan students might be interested to know of this use of gelatine. Many photographic films have a gelatine coating because no polymer has yet been developed with quite the same quality as gelatine as a coating. Some polymer alternatives can hold the light-sensitive salts and produce similar image quality, but the negatives have a shorter shelf-life. Some coated papers used for printing photographs also contain gelatine.
Although gelatine is a by-product of the meat-processing industry, it is used on a very significant scale. It is widely used in processed foods as a thickening and stabilising agent, and the quantities once required for the photographic industry were enormous. With the increasing popularity of digital photography this use of gelatine has declined, and manufacturers are looking for alternative ways of making use of their product.
If you cannot acquire suitable photographic film, you could investigate the digestion of 0.5 x 0.5 x 0.5 cm cubes of egg white or gelatine instead.
Health and safety checked, September 2008
Downloads
Download the student sheet ![]() Investigating effect of concentration on the activity of trypsin (78KB) with questions and answers.
Investigating effect of concentration on the activity of trypsin (78KB) with questions and answers.
Related experiments
Investigating an enzyme-controlled reaction: catalase and hydrogen peroxide concentration
Investigating the effect of pH on amylase activity
Investigating effect of temperature on the activity of lipase
Web links
Other enzyme activity experiments on this site, including microbial digestion of things.
http://rsc.org/Education/Teachers/Resources/cfb/enzymes.htm
Royal Society of Chemistry: Chemistry for Biologists: Enzymes
A clear and thorough presentation of information about enzymes as chemical catalysts and the factors affecting their activity.
http://eastmangelatine.com
Gelatin 101 on this site answers all the questions you might have about the production of gelatine. Other photographic companies and other gelatine production companies are available.
(Website accessed October 2011)


